
Medical imaging guides the course of much of patient care and is an essential element of biomedical research. From x-rays and ultrasound to computerized tomography (CT), functional magnetic resonance imaging (fMRI), and positron emission tomography (PET), medical imaging helps diagnose, treat, and understand a range of diseases and conditions, including cancer, cardiovascular disease, and neurodegenerative disorders.
Many Federal agencies invest in medical-imaging research or play other important roles in developing and deploying imaging technology to support clinical care and research. In response to Congressional interest, the National Science and Technology Council established in 2015 an Interagency Working Group on Medical Imaging (IWGMI) to coordinate Federal investments in medical-imaging research and develop a roadmap for medical-imaging research and development.
The IWGMI has identified “advancing high-value imaging” as an overarching theme for efforts to achieve better health outcomes and smarter health care spending through medical imaging. It has identified six objectives that should serve to guide Federal research and development activities to generate better value for patients from medical imaging and to optimize healthcare outcomes and costs:
1. Standardize image acquisition and storage. Uniform data acquisition and storage standards will allow the creation of curated, trustworthy imaging databases that enable medical-imaging research to capitalize on advances in big data and data sharing. There is broad consensus that advances in medical research and care hinge on a large data ecosystem to collect and organize vast amounts of data from patient studies and basic research. To be useful, imaging data must be trustworthy, able to be queried in a logical and consistent way, and accessible to those with legitimate need. The first step in ensuring these criteria are met is to coordinate the development and adoption of standard operating procedures for collecting, annotating, and archiving medical-imaging data. The second step is to establish approaches for curating, storing, and providing access to medical-imaging data that has been verified and validated.
2. Apply big-data and data-science concepts to medical imaging. Techniques such as machine learning, deep learning, and artificial intelligence offer considerable promise for medical imaging. Curated data sets can be used to develop and test algorithms for accurate and sophisticated machine-assisted analysis of images, identifying disease subtypes and correlations with known genetic and metabolic pathways. These new analytic approaches can help identify relationships that a human observer alone might never discover, helping improve diagnosis and suggesting optimal interventions and likely drug targets. Imaging data can be organized and presented in conjunction with other patient data in a comprehensive diagnostic “cockpit” that medical teams can use to facilitate optimal and tailored management of each patient.
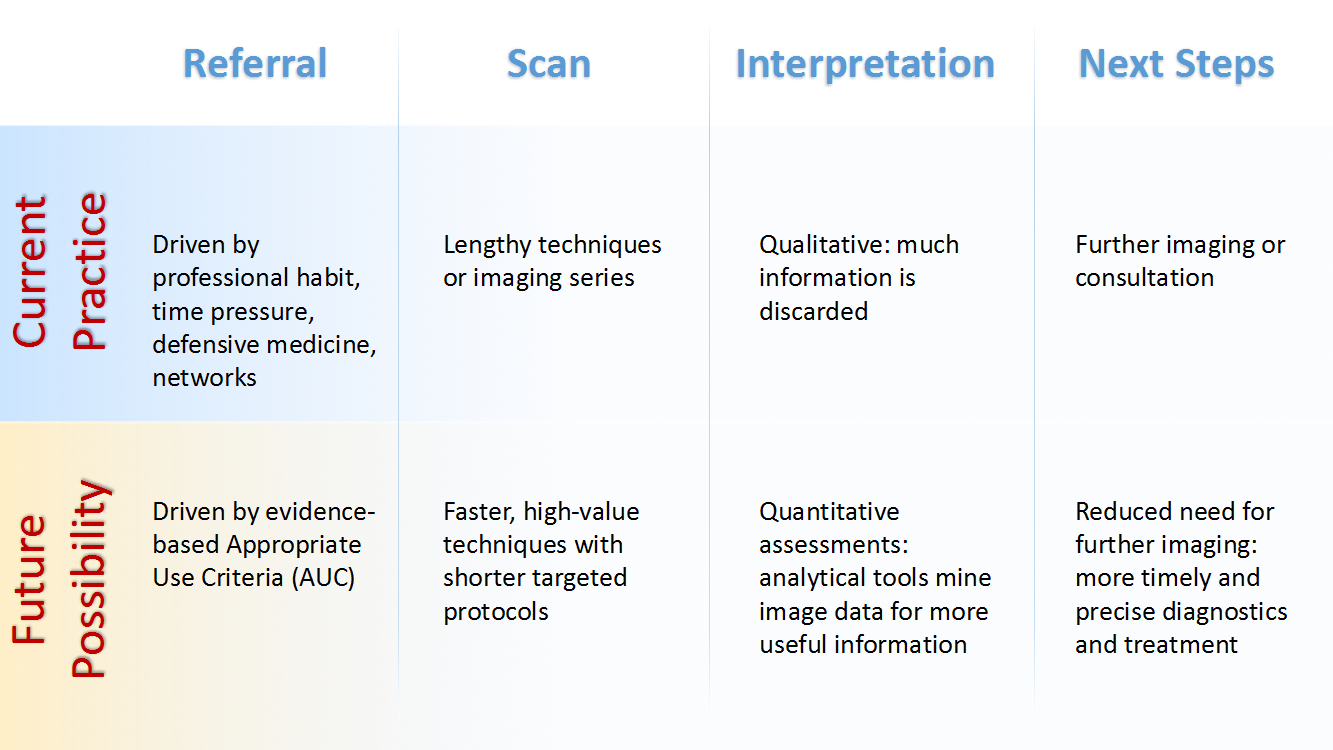
3. Get to diagnosis more quickly and cost-effectively. Streamlined medical-imaging techniques can provide quicker diagnoses and lower healthcare costs. Future research should aim to develop imaging protocols that decrease time in the scanner and improve work flow. These new protocols can be combined with quality improvements and appropriate use criteria initiatives to better target diagnostic tests for patients. Appropriate use criteria assesses whether advanced diagnostic imaging is indicated for a particular clinical scenario and, when it is, in what combination diagnostic tests should be performed.
4. Make imaging more accessible. Reducing the cost of medical-imaging equipment and increasing its portability can increase access to medical imaging for research, diagnosis, and treatment purposes. Affordability for smaller clinics and research centers gives more patients improved access to medical imaging.
5. Promulgate improved imaging practices. Technology is driving innovation in clinical decision support, but new imaging practices will require education and training so that health care professionals employ value-driven approaches. Evolving analytic software systems and technologies for sharing medical-imaging data will increase demand for new skills and training in the workforce. Priorities include disseminating knowledge about new imaging practices, developing competencies and operational skills for effective use of innovative technologies, and reorganizing workflows to improve the productivity of medical-imaging operations as practices change.
6. Improve translation of new imaging technologies. Greater collaboration and knowledge sharing among Federal agencies during the developmental stage of new medical-imaging technologies could accelerate the evaluation and approval of high-value, innovative, medical-imaging technologies and accelerate their translation from the laboratory to the marketplace—from the bench to the bedside, in medical parlance. Improved coordination among Federal bodies on their related healthcare missions may reveal new ways to best leverage limited resources.
These objectives establish goals for future medical-imaging research and related Federal activities. They reflect the IWGMI deliberations to date, drawing on information and perspectives gathered from the broad community of medical-imaging stakeholders, including patient advocacy groups, academics, professional societies, industry, and government representatives. Over the coming months, the IWGMI will develop recommended approaches for achieving these objectives and a roadmap for Federal research and development related to medical imaging. That roadmap will serve as a guide for high-value medical imaging that can improve health outcomes and health care spending.
Roderic Pettigrew is Director of the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health and co-chair of the Interagency Working Group on Medical Imaging.
Richard Cavanagh is Director of the Special Programs Office at the National Institute of Standards and Technology and co-chair of the Interagency Working Group on Medical Imaging.
Jerry Sheehan is Assistant Director for Scientific Data and Information at the White House Office of Science and Technology Policy.
Michael Cheetham is a Senior Advisor in the Office of the Director at the National Institute of Biomedical Imaging and Bioengineering.

